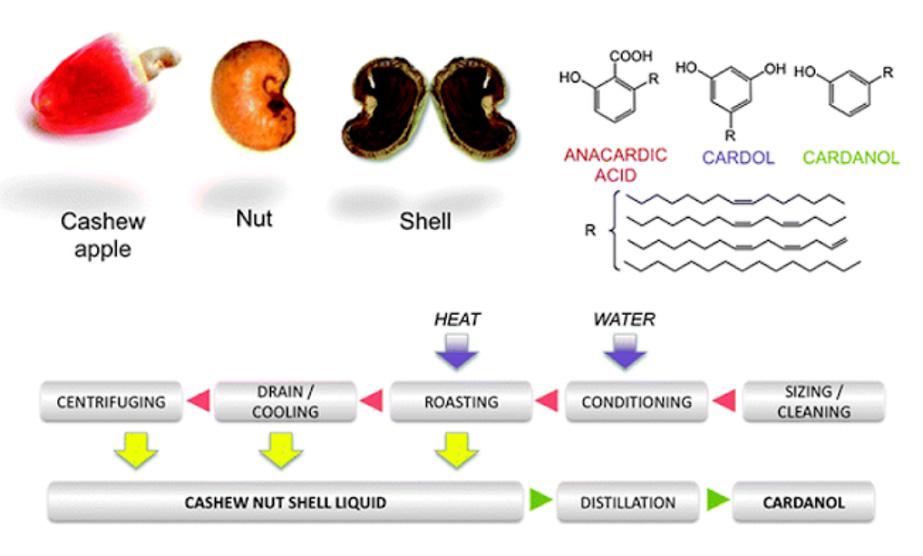
What is Cashew Nut Shell Oil?
Natural CNSL is a mixture of the alkyl phenol classes and aliphatic side chains. CNSL is a dark brown viscous liquid that causes an allergic skin rash on contact. This liquid is called natural cashew shell oil. Its English name is Cashew Nut Shell Liquid (CNSL).
CNSL is present in the composition of cashew nuts (18 - 23% of the weight of the nut) to protect the cashew kernel from insect damage. This is also a by-product of high economic and technical value obtained in cashew nut processing.
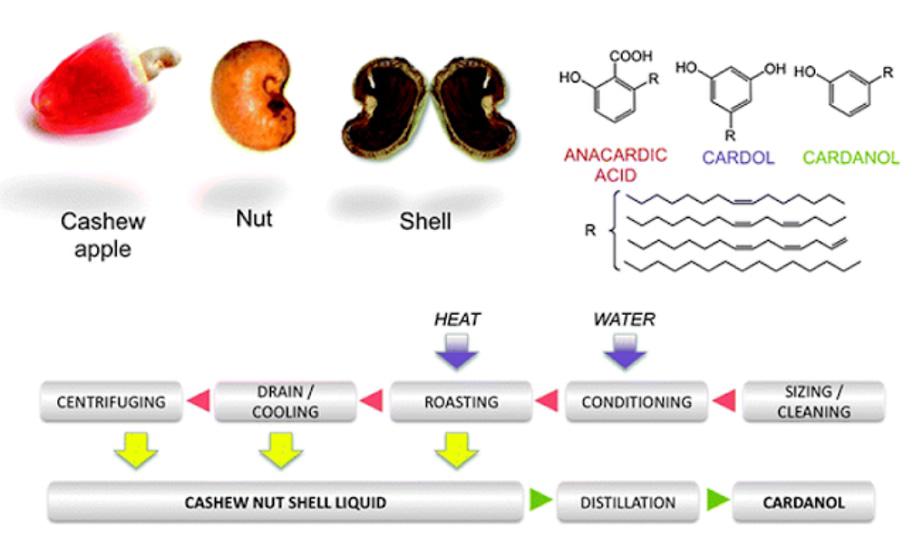
Composition and quality of cashew nut shell oil
The quality and yield of the shell oil depends on the cashew processing method. Using cold solvent extraction for n-hexane will obtain natural cashew nut shell oil (also known as extracted shell oil).
When processing cashews by the oil colander method (at high temperatures from 190 - 200 degrees Celsius), the shell oil obtained is called commercial cashew nut shell oil. There are many anacardic acid (6-alkyl salicylic acid) and cardol (3-alkyl resorcinol) in natural shell oil, while anacardic acid is decarboxylated to cardanol (3-alkyl phenol) in commercial shell oil because the oil is subjected to high temperature action. Therefore, the main components of commercial shell oil are cardanol and cardol (Ruhemann and Skinner, 1887; Spiegel and Dobrin, 1896; Smith, 1931; Pillay, 1935; Gokhale et al., 1940).
It has been clearly shown that the branched chain in anacardic acid, cardol and cardanol is not a homogeneous diolefin but a mixture of olefins with varying degrees of unsaturation (Gokhale et al., 1940; Paul and Yeddanapalli, 1954). By using modern chromatographic techniques, Murthy et al., (1968) found that cardanol (acid 2, iodine 212-228, hydroxyl 180-200) has a saturated composition of 5.4%, mono olefin 48.5%, diolefin 16.8% and triolefin 29.3%. John H. Tyman (1976) indicated that the composition of extracted cashew nut shell oil includes anacardic acid 82 +(-) 1.05%, cardol 13.8 +(-) 0.79%, 2-methyl cardol 2.6 +(-) 0.16%, cardanol 1.6 +(-) 0.17%.
Technical cashew nut shell liquid has the following composition: cardanol 83 +(-) 0.51%, cardol 14.3 +(-) 0.58%, 2-methyl cardol 2.7 +(-) 0.34%. By using thin layer chromatography, it was shown that 20% of the technical shell oils were polymerized, of which polymerized cardanol accounted for 76.35%, polymerized cardol accounts for 19.65% and polymerized 2-methyl cardol accounts for 4.22%. Therefore, the composition of typical technical shell oil has the following composition:
Cardanol: 63%
Cardol: 11%
2-methyl cardol: 2%
Anacardic acid: 1%
Polymer: 23%
Structural formula:
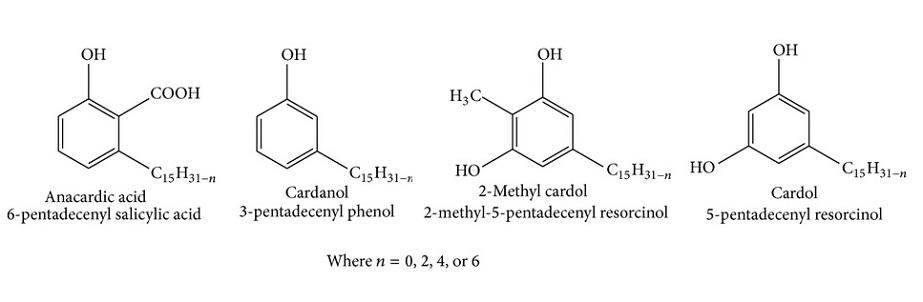
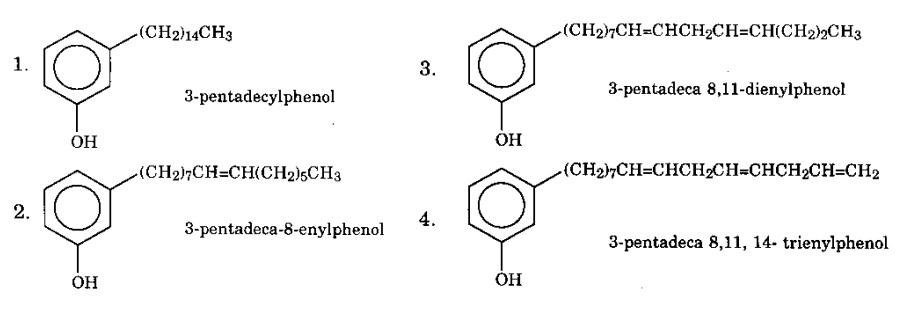
Structure of the substituent hydrocarbon chain (-CH15H31-n) in anacardic acid, cardanol, cardol as well as 2-methyl cardol
- With n = 0 we have the formula [-(CH2)14CH3]
- With n = 2 we have the formula [-(CH2)7CH = CH(CH2)5CH3]
- With n = 4 we have the formula [-(CH2)7CH = CHCH2CH = CH(CH2)2CH3]
- With n = 6 we have the formula [-(CH2)7CH = CHCH2CH = CHCH2CH = CH2]
Specifically, there can be four co-existence of cardanol compounds (Anal Chem, 1976).
The specifications of cashew shell oil
I) Technical cashew nut shell oil (untreated) according to ISO 840-1986.
1. Specific gravity 30/30 deg. C: 0.950 - 0.970
2. Viscosity at 30 deg. C, cp: 550 max
3. Moisture, % by wt (max): 1.0 max
4. Toluene insoluble, % by wt: 1.0 max
5. Loss in weight on heating, % by wt: 2.0 max
6. Ash, % by wt: 1.0 max
7. Iodine value:
a. Wijs method: 250 min
b. RK method: 290 min
8. Polymerization
a. Time, minutes: 4 max
b. Viscosity after acid washing, cp: 200 min
c. Viscosity at 30 deg. C, cp: 30 min
II) Treated cashew nut shell liquid
The extracted cashew nut shell oil is toxic to humans. Specifically, CNSL can cause skin blistering and dermatitis when it comes into contact with the skin. Before using CNSL to make plastic, it must undergo a treatment process to remove sulfur compounds and metal impurities.
This processed shell oil is called processed CNSL with the following specifications:
- Specific gravity at 25 deg. C/24 deg. C: 0.955 - 0.975
- Viscosity at 25 deg. C, cp: 800 max
- Iodine value: 240 min
- Ash, % by wt: 1 max
- Moisture, % by wt: 0.5 max
- Acid value: 14 max
- Specifications for cold pressed CNSL
Cashew shell oil is also produced by cold pressing cashew nut shell
- Specific gravity at 26 deg. C: 0.9668 - 1.0131
- Refractive index at 41.5 deg. C: 1.5158
- Saponification index: 106 - 119
- Iodine value: 270 - 290
- Acid value: 94 - 107
Features and applications of CNSL
CNSL is a dark brown viscous liquid that is toxic and causes blistering and inflammation of the skin on contact with it. CNSL does not dry in the air even if left for a long time (up to 2 years) (the iodine value of the oil does not change). CNSL is mainly composed of cardanol, cardol, 2-methyl cardol, so it does not belong to the group of glycerides like linseed oil and standard oil.
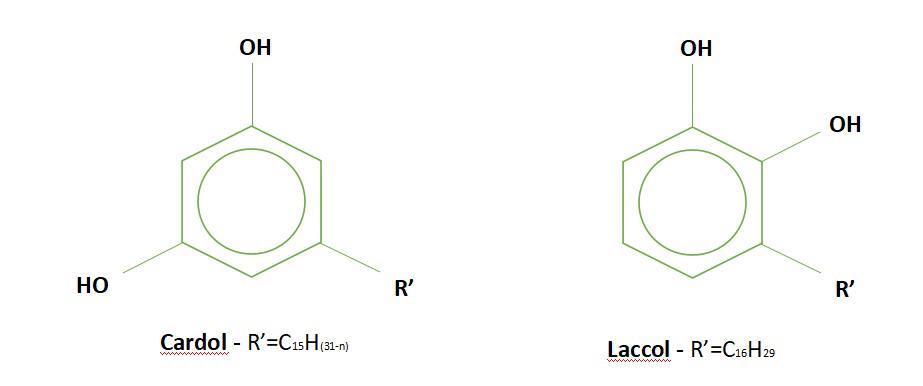
The iodine index of CNSL is higher than that of other vegetable oils. This index depends on the weather conditions at harvest as well as the growing environment of the cashew tree. The presence of cardol in cashew nut shell oil creates its toxicity. Cardol has a very similar chemical structure to the laccol found in paints.
How to neutralize the toxicity of CNSL with concentrated sulfuric acid: First, add a quantity of H2SO4 acid (d = 1.8) with a dosage of about 2.5% by weight of the shell oil and then mix and heat it up to 150 degrees Celsius. During this time, the natural compounds containing sulfides and nitrogen in the oil will be decomposed. Alternatively, it can be done in another way. First, mix a solution of cashew shell oil, water (5%) and concentrated H2SO4 (2.5%), then heat the above solution until 195 degrees Celsius to remove all sulfide gas, maintain this temperature until the seed oil reaches the required viscosity.
The dark color of cashew shell oil is caused by two components: cardol and 2-methyl cardol. CNSL's dark brown color limits its application in areas such as surface coatings or areas where colorless or light color is required. To overcome this limitation, people only use the main component of cashew shell oil, which is cardanol.
There are two ways to separate cardanol:
- Distil CNSL at a temperature of 270 degrees Celsius obtains a mixture of water. Settle this mixture into two layers, then take the oil layer and re-distill it under vacuum conditions of 10 mmHg and at a temperature of 225 degrees Celsius.
- Distillation of CNSL under vacuum pressure: conduct distillation at a temperature of 273 - 371 degrees Celsius, corresponding to a vacuum of 50 mmHg or of 232 degrees C, corresponding to a vacuum of 10 mmHg.
The obtained cardanol accounts for about 65 - 70% of the steam-distilled oil. According to the color, both cardanol and the remainder (30-35%) are used in suitable industrial fields.
The different quality of the CNSL will generate a different rate of return. CNSL in deep frying at a high temperature of 190 - 200 degrees C only obtains 40 - 50% of the yield of cardanol.
The main features of the obtained cardanol:
Condensation polymerization
At the ortho and para positions, a resol resin (with an alkaline catalyst) and a no-volac resin (with an acid catalyst) are created by the condensation of CNSL (as a common phenol) with an aldehyde. .
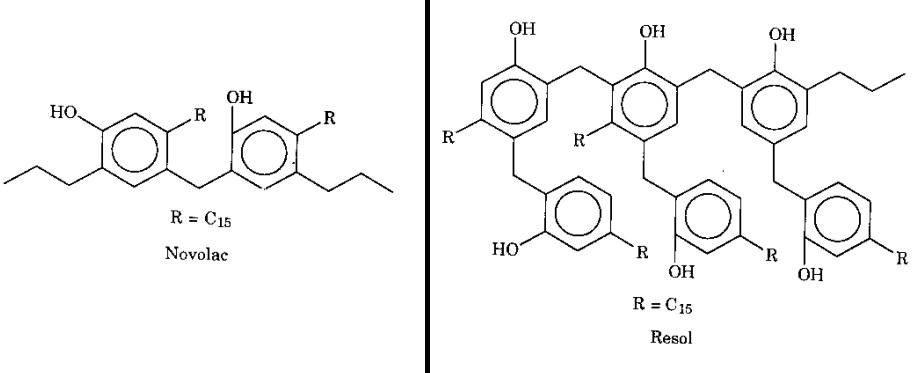
Polymers that are used to condense CNSL have many applications in creating film-forming materials such as varnishes or paints, etc. They are also considered as raw materials for the manufacture of adhesives, plastics, rubber compounds, brake linings, etc.
Oxidation polymerization
Oxidizers (such as HNO3) and oxidizing agents (with an acid catalyst) are used to oxidize CNSL. When using these oxidizers, the iodine value of the oil can drop rapidly to zero, the reaction product dries very quickly at room temperature and high temperature (Mortimer MT, 1977). This product, when dissolved in a solvent such as kerosene, turpentine or naphtha, creates a material that is very suitable for impregnating paper and canvas. Also, they used for making varnishes, paints, film-formation, etc.
Addition polymerization
CNSL can be additionally polymerized at the double bonds in the substituent by free radicals or ionic initiators. Acid catalysts such as sulphuric acid (H2SO4), hydrochloric acid (HCL), diethyl sulfate, etc. will form allylic carbon ions from the more active double bonds, thereby promoting the polymerization of CNSL (Aggar J.S, 1978).
Heating cashew shell oil with diethyl sulfate at 180 degrees Celsius for 1 hour will obtain a rubber-like product.
The application of CNSL in the preparation of brake pads is carried out as follows: First, carry out the branched polymerization reaction of CNSL and diethyl sulfate or dimethyl sulfate, then polymerize the obtained product with formaldehyde. The final product is ground into a powder.
Medal activated polymerization
When CNSL is activated by metals such as copper, aluminum, lead or their acids, hydroxides, and carbonates, it dries faster. The obtained products have high stability and good resistance to acids and alkalis. When dissolved with solvents such as naphtha, benzene or the like, these products dry quickly, adhere tightly to form a highly flexible and hard, heat resistant film.
For example, when heating a mixture of 10% copper carbonate and 90% CNSL oil with normal pressure, at 140 degrees Celsius for about 1 hour, then remove the precipitate and residual CuCO3, dissolve the mixture with naphtha, benzene or alcohol. As a result, varnish is obtained. Paper or burlap impregnated with this varnish and then dried is a good insulator for electrical coils. This varnish can also be applied directly to the coil and then dried (V. Pachai and V. Ramanathan, 1975).
Heat polymerization
In order to obtain excellent drying yeasts, it is necessary to heat CNSL with catalysts at a temperature of 160 - 180 degrees Celsius (according to Aggarwal J.S.1978). Besides, it is also possible to polymerize CNSL without a catalyst, but it takes time to raise the temperature to 230 degrees Celsius to create a polymerization reaction. During the polymerization, there is no colloidal phenomenon as in castor oil because the branched chain of cardanol does not contain the conjugated double bond in the eleostearic acid of castor oil. It should be noted that in the beginning, the polymerization rate was slow. However, after 10 hours of reaction, the reaction rate increased very quickly. The polymerization product has a very high viscosity, brown color. The products are soluble in common organic solvents such as toluene, xylene, pine oil (Luyen D. V. et al. - Journal of Chemistry T.19 N2, 1981).
CNSL has innumerable applications in polymer based chemical industries:
- Has high flexibility.
- Compatible with many other polymers.
- Can be dissolved in a variety of solvents (straight and aromatic hydrocarbon solvents).
- Improved processing capacity.
- Good wear resistance, low friction loss.
- Good heat resistance and electrical insulation.
- Good alkali and acid resistance. Does not often due to the effect of mineral oil.
- Anti-microbial, termite and insect resistant.
CNSL can create many chemical intermediates for the production of surfactants, lubricants and plasticizers for rubber, pesticides, epoxy resins, ion exchange resins, friction linings, dyes, antioxidants, accelerators, etc.
REFERENCE
Thanh D. P. (2003). Cashew nuts - Production and processing.